St. Britto Hr. Sec. School - Madurai
12th Chemistry Weekly Test - 1 (Organic Nitrogen Compounds)-Aug 2020
-
-
Assertion(A) : Aniline couples with NaNO2/HCl to give \(\beta \) -Naphthol which is a blue colour precipitate.
Reason (R) : The colour of the compound is due to conjugation of the ring.
(A) and (R) are true and (R) is the correct explanation. of (A)
Both (A) and (R) are true but (R) does not explain (A)
(A) is true but (R) is false
Both (A) and (R) are false
-
Amines have ________ odour.
carbolic;
mustard oil
fishy
garlic
-
Use of chloropicrin is as ______________
explosive
dye
anaesthetic
sterilizing agent
-
Which of the following will not undergo Hoffman's bromamide reaction ?
Ethanamide
Propanamide
Methanamide
Phenyl methanamide
-
Which of the following will not undergo diazotisation?
m-toluidine
aniline
p-amino phenol
benzyl amine
-
The basic character of amines is due to the
tetrahedral structure
presence of nitrogen atom
lone pair of electrons on nitrogen atom
high electronegativity of nitrogen
-
Positive carbylamine test is not shown by?
methyl amine
aniline
N- ethyl aniline
triethyl amine
-
Tertiary amine is less basic than secondary amine because of
delocalisation of 1t electrons
resonance effect
inductive effect
steric effect
-
Which one of the following will not undergo Hofmann bromamide reaction
CH3CONHCH3
CH3CH2CONH2
CH3CONH2
C6H5CONH2
-
How will you distinguish between primary secondary and tertiary alphatic amines.
-
-
How will you distinguish between aniline and ethylamine.
-
CH3NO2 \(\underrightarrow { \quad S{ n }_{ 2 }/HCI\quad } \) A \(\xrightarrow [ alcoholic KOH]{CHC{ I }_{ 3 } } \) B \(\underrightarrow { \quad { H }_{ 2 }/P\quad } \) Identify A,B and C.
-
-
How will you convert diethylamine into
(i) N, N - diethylacetamide
(ii) N - nitrosodiethylamine -
Identify (A}, (B) and (C)
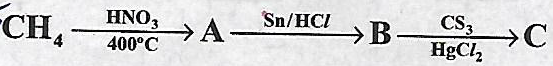
-
Complete the following the reaction.
CH3NC+2H2O \(\underrightarrow { \quad \quad { H }^{ + }\quad } \) 'A' + 'B'
-
How will convert nitrobenzene to benzoic acid?
-
An organic compound 'A' on reduction gives compound 'B' which on reaction with
trichloromethane and caustic potash forms 'C'. Compound 'C' on catalytic reduction
given N-methyl benzenamine. Identify A, B and C and write the reactions involved. -
Convert aniline to p-nitro aniline.
-
How are nitro alkanes prepared following
i) alkyl bromides
ii) methane -
-
Outline the preparation of (a) para nitroaniline from aniline, (b) tri bromo benzene from tribromo aniline.
-
Write a note on the basicity of amines.
-
-
Account for the following: (i) Nitroethane is soluble NaOH (ii) Nitroethane reacts with nitrous acid (iii) 2-methyl-2-nitro propane has neither of the properties.
-
-
Outline the mechanism of (a) Nitration of aniline, (b) Acetylation of aniline.
-
Draw a flow chart to show classification of nitro compound giving examples for each type.
-
-
Account for
i) Reduction of CH3CN gives CH3CH2NH2 while CH3NC gives (CH3)2NH.
ii) (CH3)2NH requires two molar proportion of CH3I to give the same crystalline product formed by (CH3)2N with one mole of CH3I.
iii) Nitration of aniline with conc.HNO3 may end up with same meta nitro product.
iv) p-toluidine is a stronger base than p-nitroaniline.
