St. Britto Hr. Sec. School - Madurai
12th Chemistry Monthly Test - 1 ( p-Block Elements-I )-Aug 2020
-
-
-
-
-
-
-
-
-
Identify the electron - deficient species
(BH3)2
(SiH3)2
PH3
(CH3)2
-
Which of these is not a monomer for a high molecular mass silicone polymer?
Me3SiCl
PhSiCl3
MeSiCl3
Me2SiCl2
-
Elements of group 13 mainly form covalent compounds because ______
small size
electro negativity values are high
ionization energy is very high
both (a) and (c)
-
Which one of the following compounds has similar structure to that of graphite?
Boron nitride
Boron Carbide
Aluminium Carbide
Aluminium Oxide
-
Which of the following oxide is amphoteric?
SiO2
CO2
SnO2
CaO
-
Thermodynamically the most stable form of carbon is
Diamond
graphite
Fullerene
none of these
-
The repeating unit in silicone is
SiO2

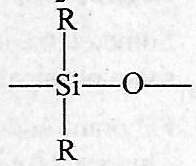

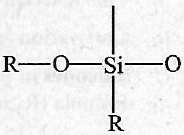

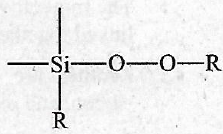
-
What are the uses of boron trifluoride?
-
What is water gas equilibrium?
-
(i) Silicones are used for making waterproof fabrics. Give reason.
(ii) Diamond - bad conductor of electricity. -
Name the building block of zeolites. Why zeolites have high porosity?
-
Name the two important ores of Boron.
-
What are silicones?
-
Write a short note on anamolous properties of the first element of p-block
-
Among group 14 elements, name
(i) the most electro negative element
(ii) the most metallic element -
How is boric acid extracted from borax?
-
What happens to CO2 when dissolved in water?
-
-
What is tetraethoxy silane? How is it obtained?
-
What is burnt alum?
-
-
What is inert pair effect?
-
What is phosgene? How is it prepared?
-
Mention the physical properties of boranes.
-
-
What are alums?
-
Give the structure of CO and CO2.
-
-
Write a note on metallic nature of p-block elements
-
-
How is CO2 manufactured?
-
Draw the structure of boric acid.
-
-
-
Describe the structure of graphite.
-
AlCl3 behaves like a lewis acid. Substantiate this statement.
-
-
Describe the structure of fullerenes.
-
What are the uses of boric acid?
-
Describe the structure of carbon nanotubes.
-
Find out the oxidation state of carbon in each of the following:
(i) CaC2
(ii) H2CO3
(iii) HCN
(iv) CO -
How is aluminium chloride prepared by McAfee process?
-
Give one example for each of the following
(i) icosogens
(ii) tetragen
(iii) prictogen
(iv) chalcogen -
Account for the following:
(i) CO is used in the extraction of metals.
(ii) CO is poisonous
(iii) CO2 is used in referigeration -
Distinguish between diamond and graphite.
-
Write a note on zeolites.
-
A double salt which contains fourth period alkali metal (A) on heating at 500K gives (B). aqueous solution of (B) gives white precipitate with BaCl2 and gives a red colour compound with alizarin. Identify A and B.
-
-
CO is a reducing agent . justify with an example.
-
How will you identify borate radical?
-
-
A hydride of 2nd period alkali metal (A) on reaction with compound of Boron (B) to give a reducing agent (C). identify A , B and C.
