St. Britto Hr. Sec. School - Madurai
12th Chemistry Monthly Test - 1 ( Hydroxy Compounds and Ethers )-Aug 2020
-
-
-
-
The reaction of ethylene glycol with Pl3 gives
ICH2CH2I
CH2=CH2
CH2=CHI
ICH=CHI
-
An organic compound 'A' reacts with methyl magnesium chloride followed by hydrolysis to form 'B' .'B' gives a blue colour with Victor Meyers test. Identify A and B respectively.
acetaldehyde, tert butyl alcohol
acetone, iso propyl alcohol
acetaldehyde, isopropyl alcohol
acetone, ethanol
-
The reaction between phenol and benzoyl chloride is the present of sodium hydroxide is namd as_______ reaction.
Cannizaro
Reimer-Tiemann
Kolbe's
Schotten-Baumann
-
Williamson's synthesis is an example of_________.
nucleophilic addition
electrophilic addition
electrophilic substitution
nucleophilic substitution
-
A compound that gives a positive iodoform test is________
1-Pentanol
2-Pentanol
3-Pentanol
Pentanal
-
Which one of the following will react with phenol to give salicyladehyde after hydrolr.sis?
Dichlo methane
trichloroethane
trichloromethane
CO2
-
Which of the following is the strongest acid?
C6H5CH2OH
C6H5OH
C6H5OCH3
CH3OH
-
Consider the following reaction
C2 H5 OH \(\underrightarrow { { PCl }_{ 5 } } \) X \(\underrightarrow { alcKOH } \) Y \(\underrightarrow { alc{ H }_{ 2 }O|{ H }^{ + } } \) Z X, Y and Z respectively are_____.
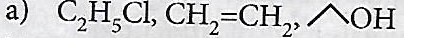
C2H4, C2H5COCl, C2H5OH

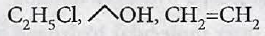
-
The IUPAC name of phenetole is_______.
ethoxybenzene
methyl phenyl ether
diethyl ether
diphenyl ether
-
(CH3)3-C-CH(OH)CH3 \(\xrightarrow { \ \\ ConH_{ 2 }{ SO }_{ 4 }\ } X\ (major\ product)\)
(CH3)3CCH = CH2
(CH3)2C = C (CH3)2
CH2= C(CH3)CH2- CH2- CH3
CH2= C (CH3) - CH2- CH2- CH3
-
RX + NaOH(aq)\(\underrightarrow { \triangle } \)ROB + NaX The above reaction proceed by_______mechanism.
nucleophilic addition
elimination
electrophilic substitution
nucleophilic substitution
-
The ultimate product obtained when glycerol reacts with oxalic acid at 533K is______.
formic acid
glycerol oxalate
allyl alcohol
acrolein
-
Give chemical test to distinguish between methanol and phenol.
-
Give the IUPAC names of
(i) CH3CH(OH)CH2OH
(ii) HO-CH2- CH2-OH
(iii) \( { CH }_{ 3 }-\underset { |\\ OH }{ CH } -{ COOH }\) -
How will you convert C2H5OH to C2H5OC2H5?
-
-
Write the structure of the aldehyde, carboxylic acid and ester that yield 4- methylpent -2-en-l-ol.
-
Arrange the following in the increasing order of their boiling point and give a reason for your ordering
(i) Butan - 2- ol, Butan -l-ol, 2 - methylpropan -2-ol
(ii) Propan -l-ol, propan -1,2,3-triol, propan -1,3 - diol, propan -2-ol
-
-
Draw the major product formed when 1 -ethoxyprop-1 -ene is heated with one equivalent of HI
-
-
What is meta merism? Give the structure and IUPAC name of metamers of 2-methyoxy propane
-
Can we use nucelophiles such as NH3,CH3O for the Nucleophilic substitution of alcohols
-
-
Arrange the following in the decreasing order of order of acid strength.
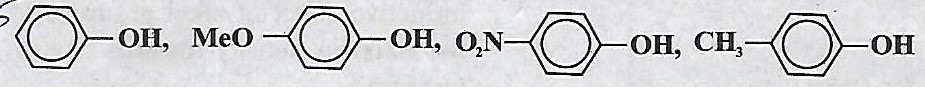
-
What is the major product obtained when two moles of ethyl magnesium bromide is treated with methyl benzoate followed by acid hydrolysis.
-
Write two uses of ethylene glycol.
-
State Saytzeff's rule.
-
What happens when crotonaldehyde is reduced in the presence of LiAIH4?
-
How will you convert of ethylene glycol to 1,4-dioxan.
-
Compare the acid strength in 1°, 2° and 3° alcohol giving reason.
-
Write the mechanism of acid catalysed dehydration of ethanol to give ethane.
-
Phenol is distilled with Zn dust followed by friedel - crafts alkylation with propyl chloride to give a compound B, B on oxidation gives (C) Indentify A,B and C.
-
How is nitroglycerine prepared from glycerol?
-
How can the following conversion be effected? (i) Phenol to phenolphthalein.
-
Account for the following: (i) Phenol has a smaller dipole moment than methanol, (ii) Phenols do not give protonation reaction readily.
-
-
Account for the following: (i) Phenol has a smaller dipole moment than methanol (ii) Phenols do not give protonation reaction readily.
-
How does glycerol react with KHSO4?
-
-
Account for the following:
(i) Phenol does not get protonated readily.
(ii) Phenol, benzene diazonium chloride, NaOH solution gives red dye.
-
Completethefollowingequations by writing the missing A,B,C,D etc.,
-
How will you prepare phenol (i) From chloro benzene (ii) From benzene sulphonic acid?
-
How is glycerol obtained commercially? State its uses.
-
Explain Swern oxidation of propan-2-ol to propanane.
-
-
Account for the following:
(a) Lower members of alcohols are soluble in water but higher members are not.
(b) Alcohols cannot be used as solvent for Grignard reagent. -
Write a note on Friedel Crafts reaction of anisole.
-
-
Distinguish between (a) Ethanol and phenol (b) Phenol and acetic acid (c) Phenol and aniline
(d) Phenol and anisole. -
What are ethers? Write note on simple and mixed ethers with examples.
-
-
Give short notes on the following:·(a) Kolbe,s reaction, (b) Riemer- Tiemann reaction
-
Starting from phenol how would you obtain the following compounds?
(a) I, 4-benzo quinone,
(b) picric acid,
(c) Anisole?
-
-
Write all possible isomers with molecular formula C4H10O and name them.
-
Explain the mechanism involved in the intermolecular dehydration of alcohols to give ethers.
