MABS Institution
11th Physics Monthly Test - 2 ( Heat and Thermodynamics )-Aug 2020
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
What is meant by Radiation? Give example.
-
Can we measure the temperature of the object by touching it?
-
A cylinder with a movable piston contains 3 moles of hydrogen at constant temperature and pressure. The walls of. a cylinder are made up of a heat insulator, and the piston is insulated by having a pile of sand on it. By what factor does the pressure of a gas increases if the gas is compressed to half its original volume?
-
Refrigerator is to maintain eatables kept inside at 9°C. If room temperature is 36°C. Calculate the co efficient of performance.
-
The temperature - entropy diagram of a reversible engine cycle is given in figure. Calculate its efficiency.
-
Write the important properties of thermal radiations.
-
-
How does the internal energy of a thermodynamic system change?
-
Calculate the heat required to convert 0.6 kg of ice at - 20°C, kept in a calorimeter to steam at 100°C atmospheric pressure, Given the specific heat capacity (Lice) of lee= 2100 Jkg-1K-1.
-
-
Describe an analytical method for determining the work done during the expansion of gas.
-
The PV diagrams for a thermodynamical system is given in the figure below. Calculate the total work done in each of the cyclic processes shown.
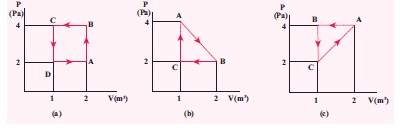
-
Explain the isobaric process and derive the work done in this process
-
Explain Joule’s Experiment of the mechanical equivalent of heat.
-
Give some examples of irreversible processes.
-
Explain Wien’s law and why our eyes are sensitive only to visible rays?
-
A gas expands from volume 1m3 to 2m3 at constant atmospheric pressure.
(a) Calculate the work done by the gas.
(b) Represent the work done in PV diagram -
Distinguish between isothermal and adiabatlc process.
