MABS Institution
11th Chemistry Weekly Test - 1 ( Haloalkanes and Haloarenes )-Aug 2020
-
-
-
-
-
-
-
-
-
-
-
-
-
Explain Sandmeyer's reaction.
-
Haloalkanes have higher boiling point and melting point than the parent alkane. Justify this statement.
-
Mention the uses of chloroform
-
Write the possible isomers for the formula C4H9Cl with structures and names.
-
Mention the uses of DDT.
-
-
What happens when alcoholic KOH is treated with (i) Ethylideue dichloride (ii) Ethylene dichloride?
-
How does bromo ethane react with the following?
(i) Silver Oxide(moist)
(ii) Sodium hydrogen sulphide
(iii) Potassium cyanide
-
-
Explain SN2mechanism with suitable examples.
-
-
An organic compound u24b6 of molecular formula C2H6O reacts with thionyl chloride in the presence of pyridine gives u24b7 C2H5Cl. u24b7 on reaction with alcoholic KOH gives ©, C2H4. ©ufe0f on treatment with Cl2 gives C2H4Cl2 as u24b9. Identify u24b6,u24b7,u24b8,u24b9 and explain the reaction.
-
Starting from methyl magnesium iodide, how would you prepare
(i) Ethyl methyl ether
(ii) methyl cyanide
(iii) methane
-
-
Answer the following.
(i) Predict the major product formed when HCI is added to iso-butylene,
(ii) What happens when CH3-Br is treated with KCN?
(iii) Identify the chiral molecule in the following pair.
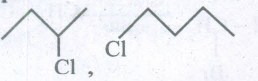
(iv) Arrange the compounds in the order of I reactivity towards SN2 displacement. 2-Bromo-2-methylbutane, I-Bromopentane, 2-Bromo pentane. -
Starting from methyl magnesium iodide how would you prepare
(i) Ethanol
(ii) 2-propanol
(iii) Tert-butyl alcohol
