MABS Institution
11th Chemistry Weekly Test - 1 ( Gaseous State )-Aug 2020
-
-
-
-
-
-
-
-
-
-
-
-
-
-
Mention the postulates of kinetic theory of gases which do not explain the behaviour of real gases.
-
Explain the graphical representation of Avogadro's hypothesis.
-
What is inversion temperature? How is it related to Vander Waals constants?
-
-
When the driver of an automobile applies brake, the passengers are pushed toward the front of the car but a helium balloon is pushed toward back of the car. Upon forward acceleration the passengers are pushed toward the front of the car. Why?
-
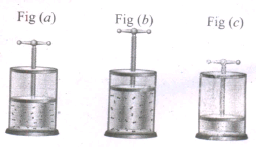
In the below figure, let us find the missmg parameters [volume in (b) and temperature in (c)]
PI = 1 atm, P2 = 1 atm, P3 = 1 atm
V1 = 0.3 dm3, V2 = ? dm3, V3 = 0.15 dm3
T1 = 200 K, T2 = 300 K, T3 = ? K.
-
-
A combustible gas is stored in a metal tank at a pressure of 2.98 atm at 25°C. The tank can withstand a maximum pressure of 12 atm after which it will explode. The building in which the tank has been stored catches fire. Now predict whether the tank will blow up first or start melting? (Melting point of the metal = 1100 K).
-
What are the observations that you get from the plot of Z vs P. (PV / RT vs P) for real gases?
-
If a gas diffuses at the rate of one-half as fast as O2, find the molecular mass of the gas.
-
Vanderwaal's constant for a gas (g) are a = 6.34 atm lit-2; and b = 52.6 ml mol-1. Find the critical temperature and critical pressure of the gas.
-
-
A flammable hydrocarbon gas of particular volume is found to diffuse through a small hole in 1.5 minutes. Under the same conditions of temperature and pressure an equal volume of bromine vapour takes 4.73 min to diffuse through the same hole. Calculate the molar mass of the unknown gas and suggest what this gas might be, (Given that molar mass of bromine = 159.8 g/mole
-
In an experimant of verification of Charle's law, the following are the set of readings taken by a student
Experiment Volume (L) Temperature (°C) 1 1.54 20 2 1.65 40 3 1.95 100 4 2.07 120 What is the average value of the constant of proportionality?
-
