MABS Institution
11th Chemistry Monthly Test - 2 ( Haloalkanes and Haloarenes )-Aug 2020
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
Explain Carbylamine reaction.(or) Give the characteristic test for primary amine.
-
What are polyhalogen compounds? Give its types with example.
-
Explain why:
(i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
(ii) alkyl halides though polar, are immiscible with water?
(iii) Gringard reagents should be prepared under anhydrous conditions? -
What happens when alcoholic KOH is treated with (i) Ethylideue dichloride (ii) Ethylene dichloride?
-
What are the uses of carbon tetrachloride?
-
Simplest alkene (A) reacts with HCl to form compound (B).Compound (B) reacts with ammonia to form compound (C) of molecular formula C2H7N.Compound (C) undergoes carbylamine test. Identify (A), (B), and (C)
-
-
Compare the bond length C-X in haloarenes and C-X in haloalkanes
-
Haloalkanes produce mixture of olefins- say true or false and justify yoyr answer,
-
-
An organic compound u24b6 of molecular formula C2H2 reacts with HCI to give C2 H4Cl2 asu24b7.u24b7on reaction with aqueous KOH will give C2H4Oas u24b8 Identify u24b6,u24b7, © and explain the reactions involved.
-
Two isomers of formula C4H9 Br are u24b6 and B, u24b6 on reaction with alcoholic KOH gives of molecular formula C4H8by E1 reaction. .u24b7 on reaction with alcoholic KOH gives u24b9 and u24ba as products by Saytzeff's rule. Identify A, B, C, D, E
-
Describe electrophilic substitution reaction of chlorobenzene with equations.
-
A simple aromatic hydrocarbon u24b6 reacts with Cl2 to give @ of molecular formula C6H5Cl.u24b7 on reaction with ethyl chloride along with sodium metal gives © of formula C8H10. © alone reacts with Na metal in the presence of ether to give © C12H10. Identify u24b6, u24b7, © & u24b9
-
Describe E1reaction mechanism with a suitable example
-
Starting from methyl magnesium iodide, how would you prepare
(i) Ethyl methyl ether
(ii) methyl cyanide
(iii) methane -
Predict the product:
(i) Chloroform + O2 \(\longrightarrow \) ?
(ii) CCl4 + H2O \(\longrightarrow \)?
(iv)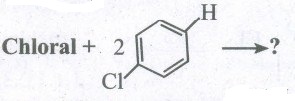
(iv) \({ CH }_{ 3 }-CH{ Cl }_{ 2 }+KOH\overset { { C }_{ 2 }{ H }_{ 5 }{ OH }_{ 3 } }{ \longrightarrow } \)?
(v) Ethylene glycol + 2PCI5 \(\longrightarrow \)?
