MABS Institution
11th Chemistry Monthly Test - 1 ( Hydrocarbons )-Aug 2020
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
Prove - All the six hydrogens in benzene are identical.
-
Cis-isomers are less stable than trans-isomers?
-
Identify A, Band C from the following equation
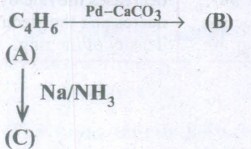
-
What is peroxide effect? Give example.
-
State Huckel's rule of aromaticity and explain it interms of cyclopentadiene, cycloxtateraene and cyclopropenylcation.
-
What happens when ealkane is,
(i) burnt in air
(ii) aromatized
(iii) reacted with steam? -
Write a brief note on polymerisation of alkenes.
-
Explain the carcinogenity and toxicity of aromatic hydrocarbons.
-
What is BHC? How will you prepare BHC? Mention its uses.
-
-
Explain how to draw the structural formula for 3-ethyl, 2, 3-dimethylpentane.
-
Explain the molecular orbirtal structure of benzene.
-
-
Write an account on the following reactions involving formation of alkane.
(i) Kolbe's Electrolytic method
(ii) Wurtz reaction
(iii) Carey - House Mechanism -
What is polymerisation? Explain with suitable example.
-
In aryl halides, halogen group is a ortho-para director and a deactivator towards electrophilic substitution reactions, why?
-
An organic compound (A) of molecular formula C2H4 which is a simple alkene reacts with Baeyer's reagent to give B of molecular formula C2H6O2• A again reacts with ozone followed by hydrolysis in the presence of zinc to form C of molecular formula CH2O. Identify A, B and C. Explain with reactions.
-
Eludicate the differences in relative stability of conformations.
-
Explain the structure of benzene.
-
Explain the acidic nature of alkynes.
-
Explain various methods of preparation of alkane.
-
Write only the equations representing the electrophilic substitution in benzene.
-
Give IUPAC names for the following compounds
1) CH3 – CH = CH – CH = CH – C ≡ C – CH3
2)
3) (CH3)3 C – C ≡ C – CH (CH3)2
4) ethyl isopropyl acetylene
5) CH ≡ C – C ≡ C – C ≡ CH -
-
Elucidate the structure of benzene in detail.
-
Explain the conformational analysis of ethane.
-
-
What are ortho-para directors? Explain why -OH group is an ortho-para director and activator.
