MABS Institution
11th Chemistry Monthly Test - 1 ( Haloalkanes and Haloarenes )-Aug 2020
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
How many isomers are possible for the formula C3H7F? Give their structures and names.
-
How does HBr react with propene?
-
Arrange the following alkyl halide in increasing order of bond enthalpy of RX
CH3Br, CH3F, CH3Cl, CH3I -
Classify the following compounds in the form of alkyl, allylic, vinyl, benzylic halides
(i) CH3 – CH = CH – Cl
(ii) C6H5CH2I
(iii) \({ CH }_{ 3 }-\underset { \overset { | }{ Br } }{ CH } -{ CH }_{ 3 }\)
(iv) CH2 = CH – Cl -
Explain why:
(i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
(ii) alkyl halides though polar, are immiscible with water?
(iii) Gringard reagents should be prepared under anhydrous conditions? -
An organic compound (A) with molecular formula C2H5Cl reacts with KOH gives compounds (B) and with alcoholic KOH gives compound (C). Identify (A),(B), and (C)
-
Explain the hydrolysis of 2-bromobutane with aqueous KOH.
-
Write the equations for the preparation of l-iodobutane from.
-
What are ogranometallic compounds? Give one example: Explain the nature of the carbon-metal bond.
-
-
Mention the uses of DDT.
-
What happens when alcoholic KOH is treated with (i) Ethylideue dichloride (ii) Ethylene dichloride?
-
-
Explain the laboratory preparation of chloroform.
-
Give two examples for
(1) gem dihalide
(2) vicinal dihalide. -
What is DDT? How is it prepared?
-
Predict the product:
(i) Chloroform + O2 \(\longrightarrow \) ?
(ii) CCl4 + H2O \(\longrightarrow \)?
(iv)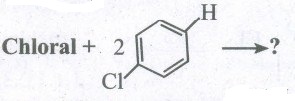
(iv) \({ CH }_{ 3 }-CH{ Cl }_{ 2 }+KOH\overset { { C }_{ 2 }{ H }_{ 5 }{ OH }_{ 3 } }{ \longrightarrow } \)?
(v) Ethylene glycol + 2PCI5 \(\longrightarrow \)? -
An organic compound u24b6 of molecular formula C2H6O reacts with thionyl chloride in the presence of pyridine gives u24b7 C2H5Cl. u24b7 on reaction with alcoholic KOH gives ©, C2H4. ©ufe0f on treatment with Cl2 gives C2H4Cl2 as u24b9. Identify u24b6,u24b7,u24b8,u24b9 and explain the reaction.
-
Explain SN2mechanism with suitable examples.
-
An organic compound u24b6 of molecular formula C2H2 reacts with HCI to give C2 H4Cl2 asu24b7.u24b7on reaction with aqueous KOH will give C2H4Oas u24b8 Identify u24b6,u24b7, © and explain the reactions involved.
-
Explain the classification of organic compounds with example.
-
Describe electrophilic substitution reaction of chlorobenzene with equations.
-
Starting from methyl magnesium iodide, how would you prepare
(i) Ethyl methyl ether
(ii) methyl cyanide
(iii) methane -
-
Answer the following.
(i) Predict the major product formed when HCI is added to iso-butylene,
(ii) What happens when CH3-Br is treated with KCN?
(iii) Identify the chiral molecule in the following pair.
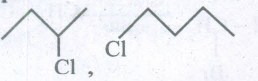
(iv) Arrange the compounds in the order of I reactivity towards SN2 displacement. 2-Bromo-2-methylbutane, I-Bromopentane, 2-Bromo pentane. -
The simplest aromatic hydrocarbon C6H6 reactsu24b7 on treatment with sodium hydroxide will (C6H5OH), Phenol, © as the product. Also Cl2 to giveu24b6 which on reaction with sodium hydroxide gives u24b7.u24b7 of molecular formula C6H6O. @ on treatment with ammonia will give C6H7N as @. Identify u24b6, u24b7, ©, and explain the reactions involved.
-
-
An organic compound u24b6 of molecular formula C3H6 react with HBr in the presence of peroxide to give C3H7Br as u24b7, u24b7on reaction with aqueous KOH gives u24b8 with molecular formula C3H5O. Identify u24b6,u24b7 and u24b8
